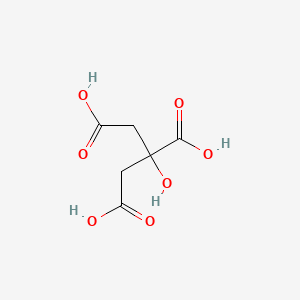
Citric Acid Ionic or Covalent
Ionic complementary polypeptides can self-assemble into stable β-sheet nanofibers through electrostatic interactions. Chemical Properties of Acid and Bases 1.

Chemical Bonds In Protein Biochemistry Notes Easy Biology Class Chemical Bond Biochemistry Biochemistry Notes
Ionic interactions involve its positively charged pyridinium ion and negatively charged phosphate group.

. Mechanism of the first phase of transamination. To find the numeric value of the acidity or basicity level of a substance the pH. Covalent compounds have weak force of attraction between their molecules so they are usually liquids or gases.
Heat protection up to 450F232C Against grooming breakage vs untreated. The acid that conducts electricity strongly is a strong acid and the acid that conducts electricity weakly is a weak acid. In acid-base reactions this usually means dissolving in water.
Ionic complementary polypeptides are chains of amino acids which can be synthesized using charged amino acid residues such as positively charged lysine K and arginine R and negatively charged glutamic acid E and aspartic acid D. What represents a formula for a chemical compound. B Ionic compounds are soluble in water but covalent compounds are insoluble in water.
The hydrogen bond has a wide transition zone which continuously merges with the covalent bond van der Waals and ionic interaction. You have arrived exactly at the right place. Salt is a substance that is produced by.
Choose all that apply. Reactions of Acids and Bases with Metals. Which of the following statements about enzymes are true.
The capacity to explain the acidic or basic character of ionic species is one advantage of the Bronsted-Lowry definition of acids and bases. The NH 2 group from the amino acid is transferred to pyridoxal phosphate with formation of the corresponding α-keto acid. Base is a bitter chemical compound that forms a water solution that turns red litmus paper to blue.
This leads to the evolution of hydrogen gas. Group of answer choices. A process that take place when an ionic compound dissolves in water allowing the ions in the compound to separate.
Builds all three types of bonds in the hair. C Ionic compounds conduct electricity when dissolved in water or when melted because they contain ions charged particles. A Enzymes are nonspecific b Enzymes speed up the rates of chemical reactions c Enzymes require a lot of energy to synthesize d Enzymes are not important in biological systems E Reactants in enzyme-catalyzed reactions are called substrates F.
Hydrogen ionic and covalent. Reduces visible signs of hair damage including split ends while preventing breakage future damage. Each atom will carry a charge from the transfer of electrons.
On the basis of the DFT calculations table S1 P-T generally has quadruple hydrogen bonds with interaction energy of 6836 kcalmol and the averaged interaction energy 1709 kcalmol is within the range of hydrogen bond of. As the term describes itself general engineering is the branch of science and technology that deals with many areas of science such as electrical mechanical chemical architectural civil and. Empirical formulae are the standard for ionic compounds such as CaCl 2 and for macromolecules such as SiO 2An empirical formula makes no reference to isomerism structure or absolute number of atomsThe term.
In chemistry the empirical formula of a chemical is a simple expression of the relative number of each type of atom or ratio of the elements in the compound. The second phase occurs by the reversal of the first phase reactions and is initiated by. PH of Acids and Bases.
Examples of the base include sodium hydroxide calcium hydroxide magnesium hydroxide etc. Compounds such as the citric acid in lemon juice the ethanoic acid in vinegar or a typical laboratory acid like hydrochloric acid all give their hydrogen ions away in chemical reactions known as acid-base reactions. The electron in each hydrogen atom is completely transferred to the oxygen atom and each hydrogen atom has a net charge of 1.
Which of the following statements is true of the bonds in a water molecule. Hydrogen ions are the basis for all acids and one definition of an acid is that it is a hydrogen ion donor. Chemical compounds are pure substances that are made up of two or more elements that are chemically combined in fixed mass ratios.
Are you a student of engineering and looking for the largest collection of engineering quiz questions for a practice test. Theorys flaws is that it doesnt account for acid-base reactions that dont involve the development of a coordinate covalent bond. A process that takes place when a covalent compound reacts with water to form new ions OR Breaking up of a molecule into charged components ions.
Examples of acid include hydrochloric acid sulphuric acid nitric acid citric acid carbonic acid lactic acid etc. When a metal reacts with an acid it generally displaces hydrogen from the acids.

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond


Comments
Post a Comment